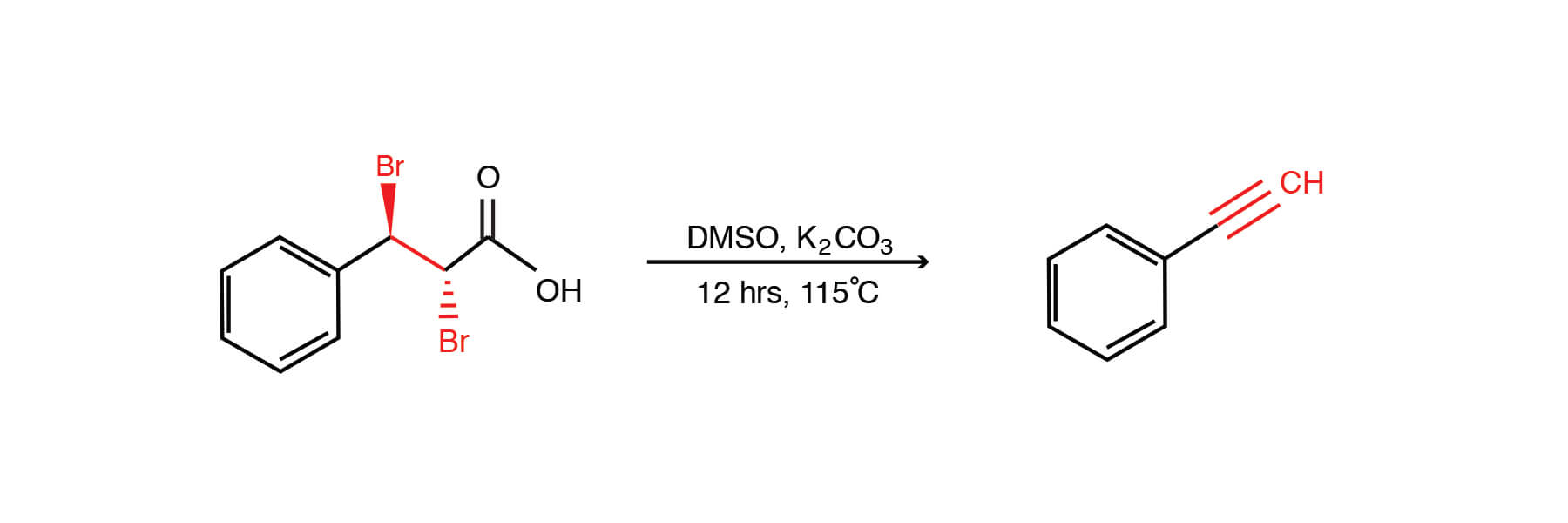
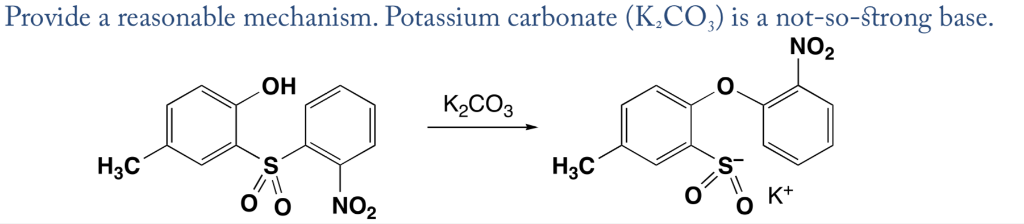
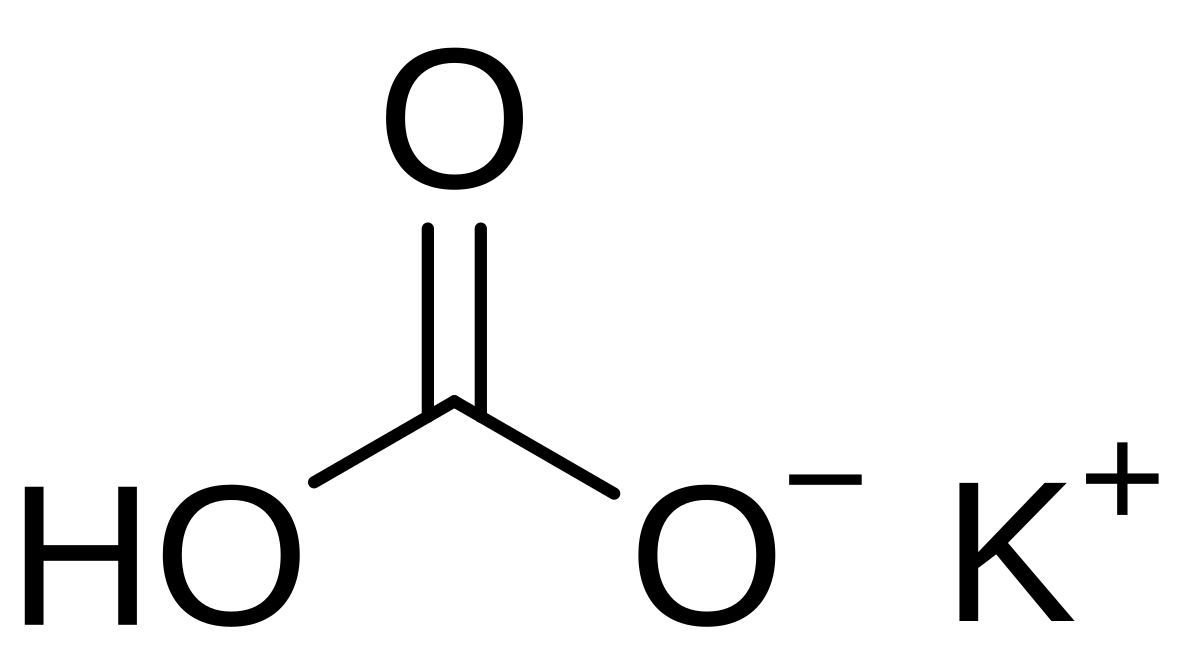
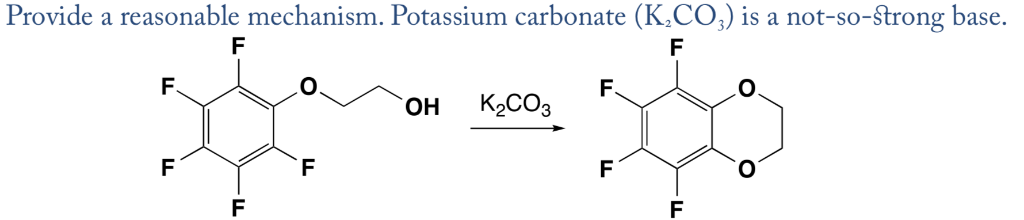
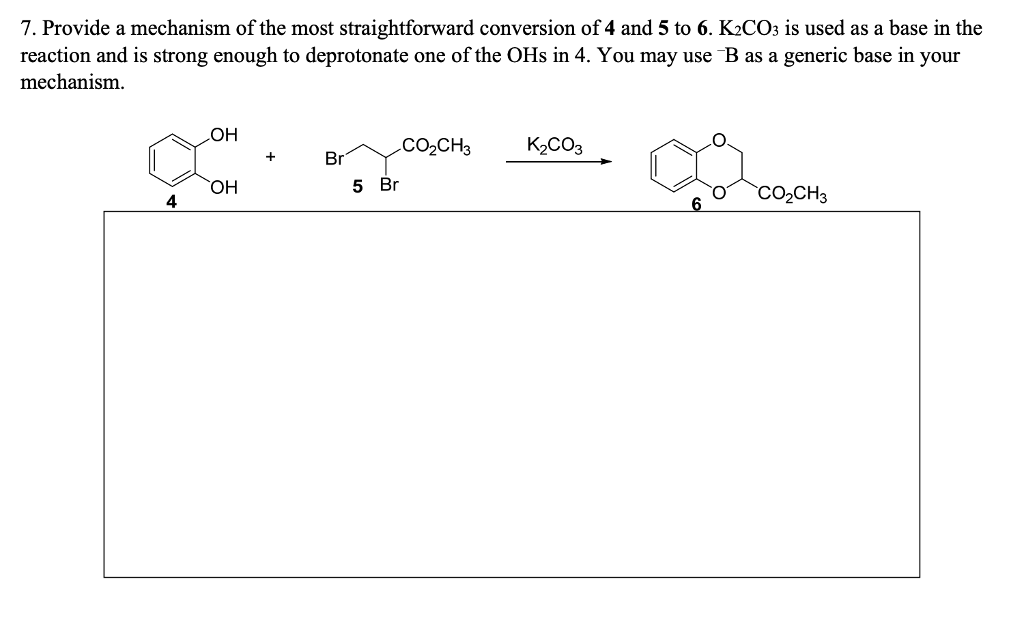
I2/K2CO3-promoted ring-opening/cyclization/rearrangement/aromatization sequence: A powerful strategy for the synthesis of polysubstituted furans - ScienceDirect
Methods for synthesizing diethyl carbonate from ethanol and supercritical carbon dioxide by one-pot or two-step reactions in the

A K2CO3‐Mediated Regioselective Synthesis of Indole/Pyrrole‐Fused 1,4‐Oxazines: An Unexpected Indole‐Fused Azlactone Synthesis - Vandavasi - 2014 - European Journal of Organic Chemistry - Wiley Online Library

Potassium carbonate as a base for the N-alkylation of indole and pyrrole in ionic liquids - ScienceDirect

Proposed schematic illustration of the K2CO3-Gly DES formation mechanism | Download Scientific Diagram

K2CO3 Promoted Cascade Reaction for the Preparation of 1H-Imidazol-4- yl-1-amine Derivatives | Bentham Science
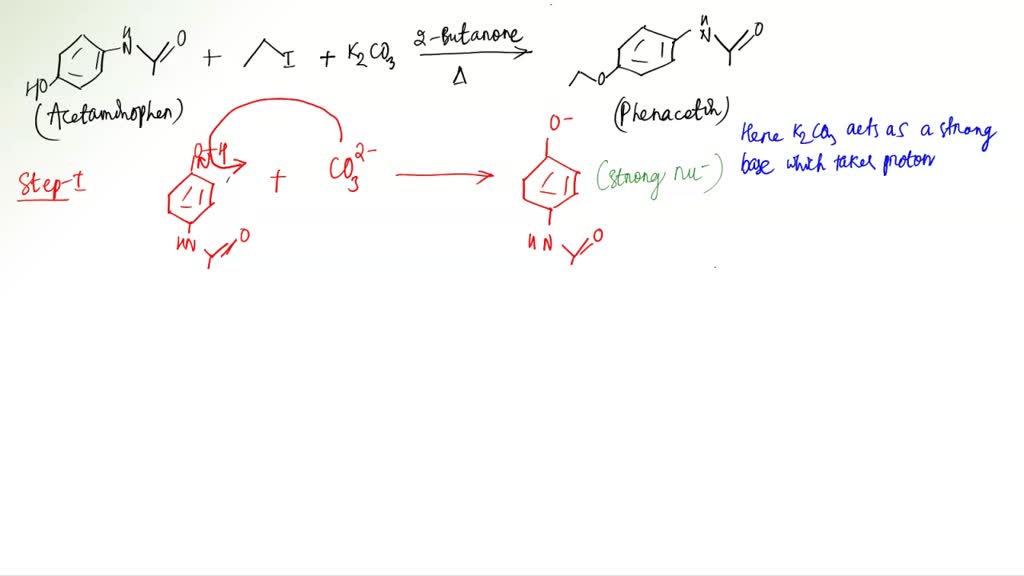
SOLVED: Outline and then draw and describe the mechanism for the Williamson ether synthesis of acetaminophen and iodoethane in the presence of potassium carbonate using the curved-arrow formalism (See Scheme 3) to
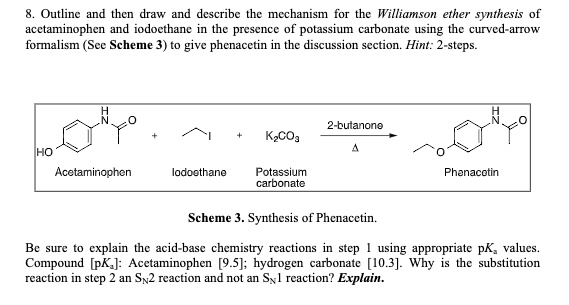
SOLVED: Outline and then draw and describe the mechanism for the Williamson ether synthesis of acetaminophen and iodoethane in the presence of potassium carbonate using the curved-arrow formalism (See Scheme 3) to